Inorganic chemistry studies the reactions and behaviors of non-organic and organometallic compounds. It addresses compounds that are not carbon-based, though some inorganic compounds may contain some carbon. Inorganic chemistry has applications throughout the chemical industry.

In Depth: What Is Inorganic Chemistry?
Now, we expand on the general definition in our introduction. We know that organic chemistry is the science that explores carbon-based molecules. Logic dictates that inorganic chemistry explores substances with little to no carbon.
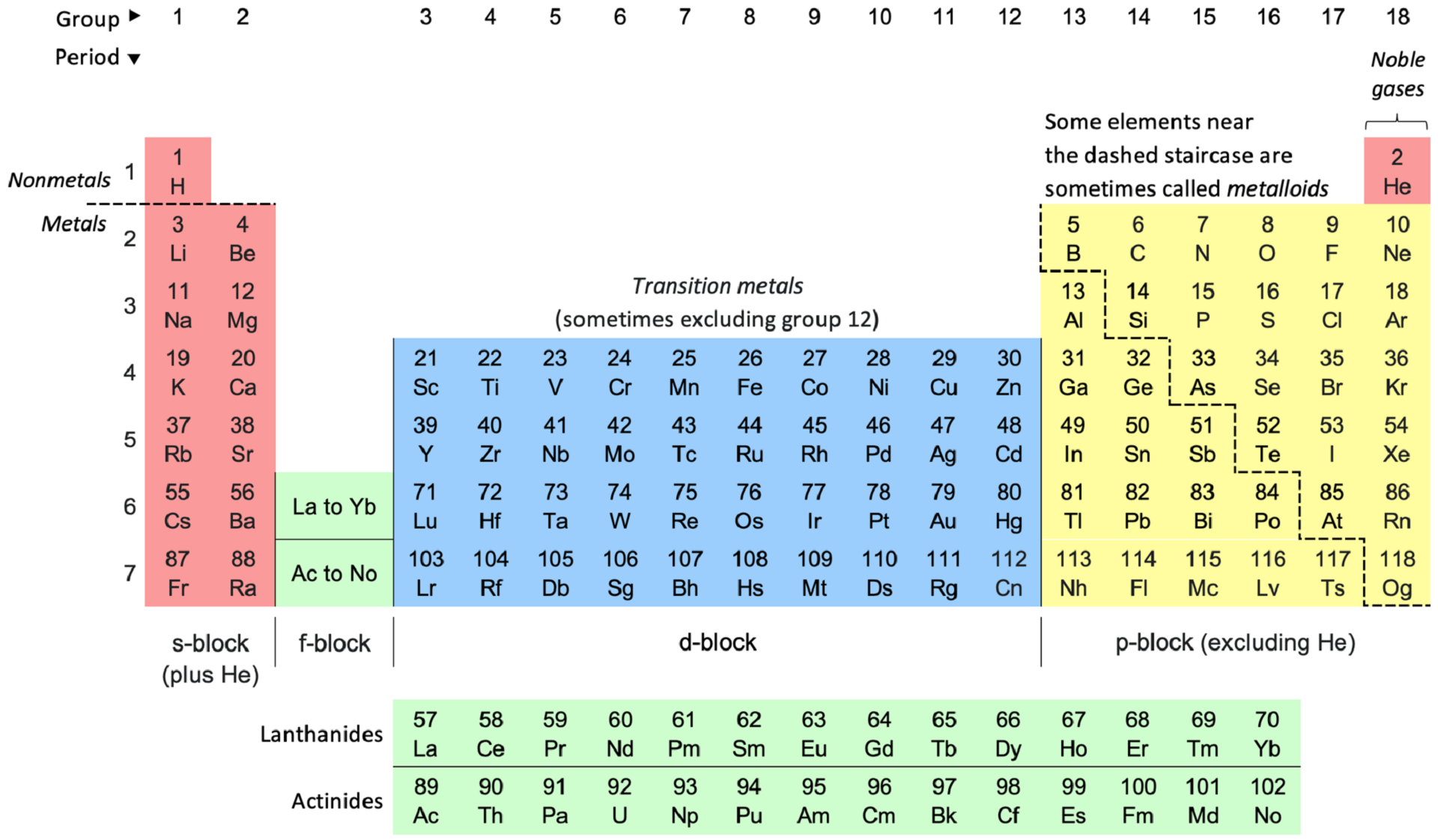
The periodic table of elements gives us an easy guide to inorganic elements. It marks the line between metals and non-metals, and draws a 'staircase' to identify metalloids. More specifically:
- The S-block: electropositive metals (except hydrogen and helium)
- The P-block: distinctively metallic or non-metallic elements
- The D-block: metals with many oxidation states
- The F-block: difficult to distinguish metals, due to their similarity
Within this framework, particular aspects of inorganic chemistry work emerge.
Inorganic Chemistry Subfields
Organometallic Chemistry
- metal-carbon bonded compounds
- class includes boron silicon selenium
- deals with organic synthesis of compounds
Bioinorganic Chemistry
- relevant to medical chemistry
- deals with metal-containing biomolecules
- examples: enzymes, oxygen transport, and others
Cluster Chemistry
- explores compounds with multiple metals
- particularly with bridging ligands or metal-metal bonds
Materials Chemistry
- exploring materials' properties
- vital to nanomaterials' research
Inorganic chemistry has wide industrial applications, including traditional ones such as making cleaning compounds. The tech sector has always been at the forefront of inorganic chemistry exploration, from creating the first lightbulbs to today's nanotubes.

How Did Inorganic Chemistry Become a Discipline?
As much overlap exists between the inorganic and organic chemistry study fields, it took some time to establish inorganic chemistry as a separate field. Even now, high school studies include inorganic topics in general chemistry studies.
But, as always happens when knowledge grows, one area of study breaks away, to become its own discipline. For inorganic chemistry, it was a rather muted break and not a complete one. It came rather late, too: just after the 20th Century began.
The Inorganic Chemistry Trailblazer
Besides these and other discoveries, Mr. Werner's work influenced many other scientists. We note these two, in particular:
Abegg's Rule
- The difference between max positive and negative valences is eight.
- Author: Richard Abegg
The Octet Rule
- Each valence tends to have eight electrons in its valence shell.
- Author: Gilbert N. Lewis
Friedrich Wilhelm Ostwald, the scientist who shaped the discipline of physical chemistry, might have been a reluctant chemist. By his own admission (and academic record), mastering chemistry was never on his radar, either as a child or a student.
By contrast, Alfred Werner always knew he wanted to study science. His college had no doctorate program, so he relocated to Paris, to a school that did. Toward the end of his career, he returned to Switzerland, to teach the next generation of chemists.

Studying Inorganic Chemistry
We don't see a distinction between inorganic chemistry and studies of other types of chemistry until the college level. Then, one must apply to a graduate program to specialize in inorganic chemistry. Undergraduates will study general chemistry to gain access to those higher-level courses.
High school level learning doesn't offer inorganic chemistry courses distinct from general studies, either.
High School Inorganic Chemistry
High school level learners will study seven areas relevant to inorganic chemistry. Group 1 elements, alkali metals, is their first topic.

These metals form the left-most column on the periodic table (excluding hydrogen). Among their characteristics: they're very soft, have low densities, and are very reactive.
Group 7 elements are next. These halogens are toxic and non-metallic. You'll also study the noble gases: helium, argon, xenon, and neon, among them.
With these fundamentals mastered, students discover reactivity and uses of these metals, and their reactants.
Students will conduct experiments, and report their findings. These lab tests will be elementary, but fundamental to understanding the science. These experiments include:
- flame tests: subjecting various metals to flame, to observe their change in colour
- sodium hydroxide tests: observe various metal ions' reactions to hydroxide ions
- anions tests: negatively-charged substances exposed to reactants
- tests for common gases: expose gases to various catalysts; observe their reactions
In most respects, high school inorganic chemistry is more of a memory exercise than practical learning. Your exam papers will challenge you to recall test procedures and results. At this level of your inorganic chemistry studies, problem-solving and visualising plays a minor role.
College Inorganic Chemistry
College students begin their freshman year by revisiting their high school chemistry topics. They will explore the periodic table in-depth, drilling into the table's structure, the structures' significance, and why the elements are so positioned. They will then expand on their earlier learning on metals and metals' reactions.
At this stage, students begin to see the interconnections that underpin chemical science. The methodical process of exposing students to chemistry fundamentals ensures understanding of elementary concepts.
Reinforcing that knowledge before introducing new topics prepares you to grasp more complex ideas, later on.
Still, at this stage of learning, much of your work in chemistry mastery revolves around memorizing facts. Memorizing is easier if you understand the concepts, though.
Mastering Chemistry Concepts
Should you need help making sense of inorganic chemistry concepts, a few sessions with a private tutor will help. Superprof chemistry tutors can help you organize information, to better make sense of it.
Without getting too deeply in the weeds, your Supeprof tutor will highlight important chemistry information. The type of knowledge you need to succeed in your exams.

Inorganic Chemistry Careers
Inorganic chemistry is essentially the study and creation of synthetic compounds. So, inorganic chemistry degree holders might see only manufacturing jobs in their future. They have far more options than they think.
In fact, some of today's hottest careers revolve around inorganic chemistry.
Food Production
These days, we hear a lot about chemical additives in our food. It's a controversial - downright scary topic, but these ingredients are often necessary for food safety and preservation.

After all, pressed for time as we are, we no longer have the luxury of home-cooked meals made with fresh ingredients. Today's food convenience is wholly due to chemistry. And, in large part, to inorganic chemistry, as these two examples demonstrate.
Sodium bisulfate
- Molecular formula: NaHSO4
- an acid salt used in many foods and drinks
- also called bisulfate of soda (and other names)
Sodium nitrate
- Molecular formula: NaNO3
- an alkali metal nitrate salt used on meats
- also called Chile saltpeter
These and other salts keep our foods safe to eat for longer. Flavor enhancers and aroma boosters (ammonia chloride, for example), help our foods satisfy our senses.
The agricultural industry is looking for innovators to formulate the next generation of safe fertilisers and pesticides.
As the inorganic chemist on staff at a large agro-chem firm, you will ensure the right measures of chemicals to treat the foods. You'll also test them as a quality control measure.
Or, you may keep to the research and development aspects of food production. In all cases, you will likely work on a team with a background in biochemistry studies.
Green Technology
Today's headlines scream about 'rare earths', minerals with a growing list of applications. Inorganic chemists have their hands full with juggling production demands and discovering new ways to extract and purify them.
These minerals already feature in our smartphones and tellies, computers and other electronic devices. They are essential for radar and military applications. The Green Revolution needs them to make car batteries and wind turbines, among other uses.

Chip Wars
When headlines aren't full of rare earths, computer chips and semiconductors take the lead. The race to make chips smaller, more capable, and more efficient is running full-out. And that's on top of streamlining and increasing production.
Inorganic chemists are working double-time to innovate production. They come up with novel ways to refine, purify, and combine rare earths to maximise their potential.
Inorganic chemists are working double-time to innovate production. They come up with novel ways to refine, purify, and combine rare earths to maximise their potential.
Of course, work in general manufacturing concerns remains an option. Chemical manufacturing companies are always looking for new talent. Among such firms, we find big name like:
- BASF
- Dow Chemical
- Unilever
- Dupont
- Mitsubishi Chemical
- LG Chem
- Formosa Plastics
- Ineos
- Linde
Whichever direction you plan to take your career in, you now know you have more options to consider. Far more choice than, say, those who earned their degree in analytical chemistry.
So, if you were considering giving up on inorganic chemistry, think again. This field of study needs talented people like you! 😎
Summarize with AI:















